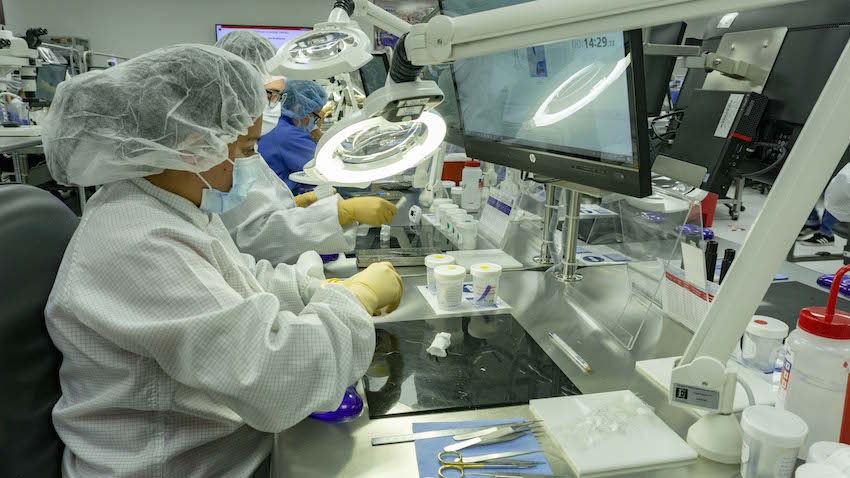
Currently, the company produces some parts of these devices there, but the finished product will soon be exported to be used in different medical centers around the world.
This operation currently generates 530 jobs and will exceed 1,000 jobs when it operates at 100%.
To get to know this California-based Company’s operation in the country more deeply, INversion INmobiliaria (IN) magazine spoke to Josue Campos (JC), the General Manager designated to lead the launching and consolidation in Costa Rica.

The meeting was held at the Company’s modern facilities, comprised by two buildings that make up 26,000 square meters in total.
The superb and environmentally friendly design is equipped with cutting edge technology and welcoming amenities, to offer the greatest hospitality to its employees.
Also, special attention was placed on logistics, providing five platforms with three receiving positions and two shipping floors, strong decks and high ceilings for vertical product stockpiling.
If you are interested in finding out why this Company focused its efforts toward Costa Rica, and specifically, toward La Lima in Cartago; or on the employee profile it is looking for, we invite you to read the full interview.
IN: What does Edwards Lifesciences produce in Costa Rica?
JC: The current focus in Costa Rica is to support the Edwards’ global heart implantable device strategy. Today, we are manufacturing heart valve replacement sub-assemblies and, with the new facility, we will be able to build heart valves from start to finish, which will be completely manufactured in Costa Rica and then exported and used in medical centers around the world.
IN: Why did Edwards decided to settle in the country?
JC: Edwards’ business has continued growing year after year, thus creating the need to be capable of expanding its manufacturing capacities to keep saving lives around the world. After a detailed research, Edwards chooses Costa Rica for its excellent commercial and political environment, skilled workforce, and broad experience in the production of medical devices.
IN: Why did the Company specifically choose Cartago, as its Operations Center?
JC: It was a combination of having the opportunity to design and build a manufacturing site to meet the specific needs of new products, in a new Free Trade Zone Park (La Lima), the proximity to Technical Universities, and being able to benefit from the great talent the Cartago area offers, while creating new job opportunities at the same time.
IN: Does Costa Rica provide foreign countries with an adequate accompaniment to make it easier for companies to enter the country?
JC: From day one, Edwards has received unconditional support from the Costa Rican Investment Promotion Agency (CINDE, for its Spanish acronym) and the Special Economic Area of Cartago (ZEEC, for its Spanish acronym). Not only in the process of understanding what is required to establish the operation, but also the accompaniment process to make that operation grow.
IN: Which are the main features of Edwards’ facilities in La Lima, Cartago, and what amenities do its facilities offer?
JC: The new building has a 20,000 square meter area, and it was built taken sustainable design and building practices into consideration to achieve the LEED certification; it was projected to hold six production clean rooms, a warehouse with the capacity to hold five platforms (three for receiving and two for shipping), Chemical and Microbiology laboratories, different conference rooms, technical training areas with classrooms for training, a mezzanine with office areas for management personnel, and another mezzanine for utility equipment that is protected within the facility, to simplify its maintenance, and allowing access to servicing without the need to enter or impact manufacturing clean rooms. This includes 461 parking spots for automobiles and motorcycles, and covered bus stops that connect with the building for our employees.


IN: What amenities does the building offer to employees in terms of entertainment and to be hospitable, considering it is there where they spend most part of the day?
JC: EThe building design considered the capacity to provide areas to facilitate and support the balanced development of our employees. Most of the office areas have outside views; additionally, we have:
⦁ A full kitchen cafeteria and space to sit 520 individuals, including an independent area for coffee, service isles and an open terrace.
⦁ An Asociación Solidarista office.
⦁ Wide hallways and two elevators to connect with the mezzanines which may support personnel recruitment strategies for individuals with some disabilities.
⦁ Private offices, large open cubicles, and work team collaboration areas.
⦁ A medical clinic and nursing rooms.
⦁ Physical wellness area / Fitness gym with free-weight equipment, multi-purpose machines and a cardiovascular focused workout area.
IN: Why did the Company decide to build its building under the “built to suit” concept?
JC: The building design considered the capacity to include all the improvements and recommendations from our global operations, such as being able to support the future growth strategy in terms of manufacturing, including personnel and material flow analysis. Basically, it was a design created by a team of Edwards leaders and engineers (focused on the needs of the heart implant operation) and by talented Costa Rican engineers and architects from companies such as Garnier andamp; Garnier, Termoaire, and Circuito, among others.
IN: What was the Company’s investment in its Cartago facilities?
JC: The current investment on the new building is of over $100 million.
IN: What percentage of the Company’s production will be carried out from Cartago?
JC: Today, we will have two production clean rooms with a capacity to manufacture transcatheter heart valves and surgical valves. The success we have in this startup will allow us to be capable of building the